CasNo:80-05-7
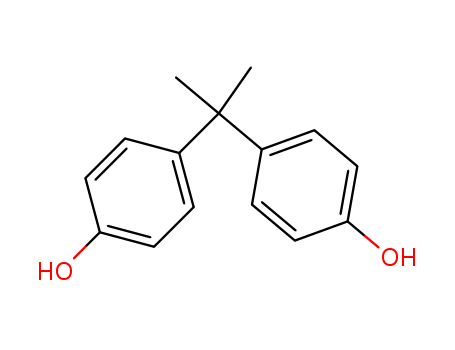
Product Name:Bisphenol A
Molecular Formula:C15H16O2
Appearance:White to light brown flakes or powder
Purity:99%
Buy High Quality Bisphenol A 80-05-7, Sale On Stock
- Molecular Formula:C15H16O2
- Molecular Weight:228.291
- Vapor Pressure:<1 Pa (25 °C)
- Melting Point:158 to 159 °C (430 K)
- Refractive Index:1.5542 (estimate)
- Boiling Point:220 °C (493 K) / 4 mmHg
- PKA:10.29±0.10(Predicted)
- Flash Point:227 °C
- PSA:40.46000
- LogP:3.42370
Bisphenol A(Cas 80-05-7) Usage
|
Uses |
Bisphenol A (BPA), with CAS number 80-05-7, is a widely used chemical compound with diverse industrial applications, particularly in the production of plastics and related materials. It has been associated with concerns related to occupational sensitization and environmental releases, although its presence in the environment is generally low due to its rapid degradation and low bioavailability. |
|
Description |
Bisphenol A (CAS 80-05-7) exists in the form of white or tan crystals or flakes with a mild phenolic odor. It has a very low vapor pressure and is mildly soluble in water. It has been reported as an allergen in various occupational settings, including fiberglass production, semisynthetic waxes, footwear manufacturing, and dental materials. Bisphenol A is known for its estrogenic action. In experimental animal studies, low-dose in utero exposure to bisphenol A has been associated with morphological changes in the vagina of postpubertal offspring. |
|
Hazard |
Poison; moderately toxic; teratogen; irritant. |
|
Health Hazard |
Dusts irritating to upper respiratory passages; may cause sneezing. |
|
Flammability and Explosibility |
Notclassified |
|
Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required. |
|
Incompatibilities |
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides and acid anhydrides. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
80-05-7 Relevant articles
Selective synthesis of Bisphenol-A over mesoporous MCM silica catalysts functionalized with sulfonic acid groups
Das, Debasish,Lee, Jyh-Fu,Cheng, Soofin
, p. 152 - 160 (2004)
Mesoporous MCM-41 and -48 silicas anchor...
ZnCl2-modified ion exchange resin as an efficient catalyst for the bisphenol-A production
Wang, Bao-He,Dong, Jin-Shi,Chen, Shuang,Wang, Li-Li,Zhu, Jing
, p. 1423 - 1427 (2014)
A ZnCl2-modified ion exchange resin as t...
Hydrolysis of polycarbonate in sub-critical water in fused silica capillary reactor with in situ Raman spectroscopy
Pan, Zhiyan,Chou, I-Ming,Burruss, Robert C.
, p. 1105 - 1107 (2009)
The advantages of using fused silica cap...
Synthesis, characterization, and catalytic activity of sulfonic acid-functionalized periodic mesoporous organosilicas
Yang, Qihua,Liu, Jian,Yang, Jie,Kapoor, Mahendra P.,Inagaki, Shinji,Li, Can
, p. 265 - 272 (2004)
Sulfonic acid-functionalized periodic me...
Optimization of process parameters for preparing a solid catalyst for bisphenol synthesis
Kozlova,Tereshchuk,Myznikov,Antonenko,Zubritskaya,Bazanov
, p. 406 - 413 (2016)
The results of optimization of the proce...
Photocatalytic Degradation of 4,4′-Isopropylidenebis(2,6-dibromophenol) on Magnetite Catalysts vs. Ozonolysis Method: Process Efficiency and Toxicity Assessment of Disinfection By-Products
Balawejder, Maciej,Barylyak, Adriana,Bobitski, Yaroslav,Kisa?a, Joanna,Tomaszewska, Anna
, (2022/03/31)
Flame retardants have attracted growing ...
Boosting the methanolysis of polycarbonate by the synergy between ultrasound irradiation and task specific ionic liquids
D'Anna, Francesca,Sbacchi, Maria,Infurna, Giulia,Dintcheva, Nadka Tz.,Marullo, Salvatore
supporting information, p. 9957 - 9967 (2021/12/24)
In an attempt to perform polycarbonate c...
80-05-7 Process route
-
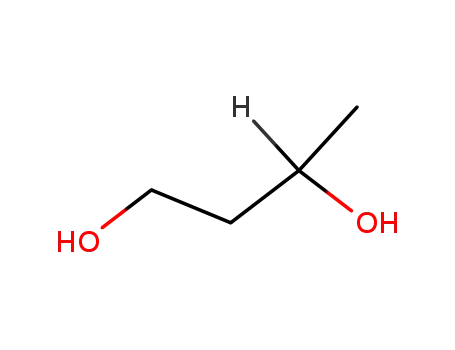
- 18826-95-4,107-88-0
1.3-butanediol

-
![4-methyl-[1,3]dioxan-2-one](/upload/2023/8/e7f2697c-0f93-4c3b-a51c-b1eef0b0433b.png)
- 17361-58-9
4-methyl-[1,3]dioxan-2-one

-
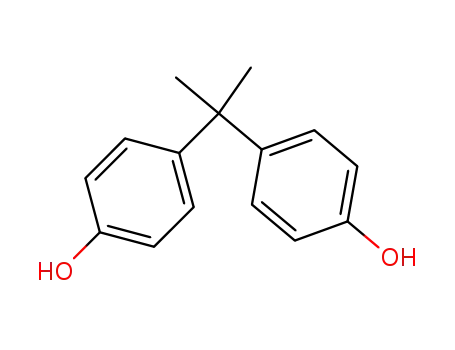
- 80-05-7
BPA
| Conditions | Yield |
|---|---|
|
With zinc(II) oxide; tetrabutyl-ammonium chloride; In tetrahydrofuran; at 100 ℃; for 7h; under 760.051 Torr; Autoclave; Inert atmosphere;
|
-
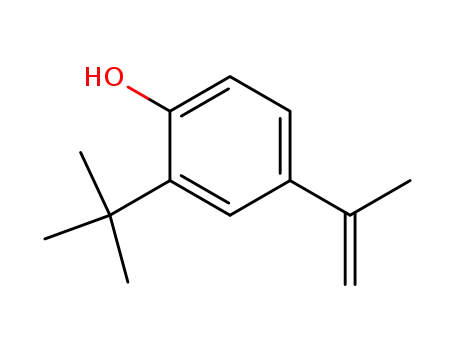
- 32565-67-6
2-tert.-Butyl-4-isopropenyl-phenol

-
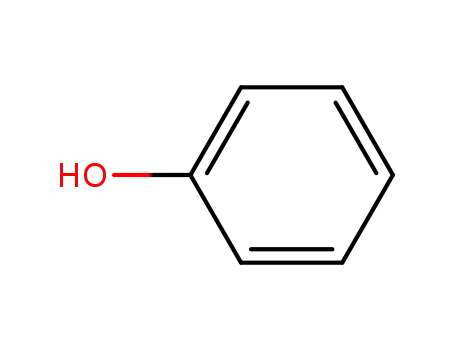
- 108-95-2,27073-41-2
phenol

-

- 80-05-7
BPA

-
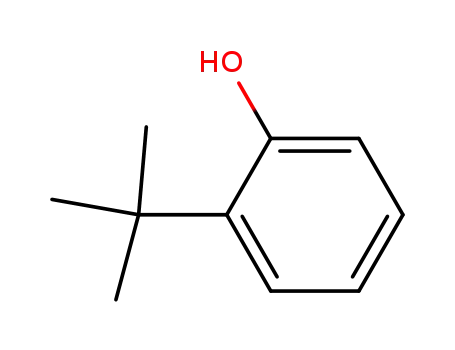
- 88-18-6
2-tert-Butylphenol

-
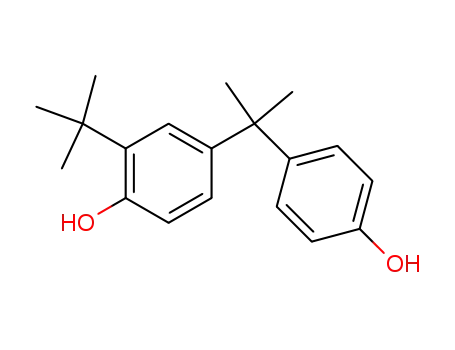
- 19546-14-6
2-tert.-Butyl-4,4'-isopropyliden-bis-phenol
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In toluene;
|