CasNo:782487-28-9
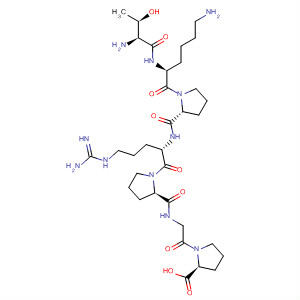
Product Name:Semaglutide
Molecular Formula:C20H35NO2
Purity:99%
Chinese Factory Supply Wholesale Semaglutide 782487-28-9 with Safe Shipping
- Molecular Formula:C20H35NO2
- Molecular Weight:321.5
- Boiling Point:451.6±45.0 °C(Predicted)
- PKA:15.05±0.10(Predicted)
- PSA:66.48000
- Density:1.07±0.1 g/cm3(Predicted)
- LogP:3.80370
Semaglutide (Cas 782487-28-9) Usage
Semaglutide is primarily excreted through the kidneys, and to a lesser extent, in the feces. In a different formulation, Semaglutide is approved for weight management in individuals who are overweight or obese. It is used to help promote weight loss and improve overall metabolic health. Semaglutide mimics the action of glucagon-like peptide-1 (GLP-1), a hormone released by the gut in response to eating. Some GLP-1 receptor agonists, including Semaglutide, have demonstrated cardiovascular benefits, reducing the risk of major adverse cardiovascular events (MACE). This makes Semaglutide a valuable option for people with type 2 diabetes who are at high risk of cardiovascular issues.
782487-28-9 Relevant articles
Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial
Helena W Rodbard, Ildiko Lingvay, John Reed, Raymond de la Rosa, Ludger Rose, Danny Sugimoto, Eiichi Araki, Pei-Ling Chu, Nelun Wijayasinghe, Paul Norwood
, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 6, June 2018,
At week 30, mean HbA1c reductions [mean baseline value, 8.4% (67.9 mmol/mol)] with semaglutide 0.5 and 1.0 mg were 1.4% (15.8 mmol/mol) and 1.8% (20.2 mmol/mol) vs 0.1% (1.0 mmol/mol) with placebo [estimated treatment difference (ETD) vs placebo, –1.35 (14.8 mmol/mol); 95% CI, –1.61 to –1.10 and ETD, –1.75% (19.2 mmol/mol); 95% CI, –2.01 to –1.50; both P < 0.0001].
Pharmacokinetics, Safety and Tolerability of Oral Semaglutide in Subjects with Renal Impairment
Charlotte Granhall, Flemming L. Søndergaard, Mette Thomsen & Thomas W. Anderson
, Clinical Pharmacokinetics 57, pages1571–1580 (2018)
Subjects were categorised as having normal renal function (n = 24), mild (n = 12), moderate (n = 12) or severe (n = 12) renal impairment, or end-stage renal disease (ESRD) requiring haemodialysis (n = 11) and received once-daily oral semaglutide (5 mg for 5 days followed by 10 mg for 5 days) in the fasting state, followed by 30 min fasting after dosing.