CasNo:96-26-4
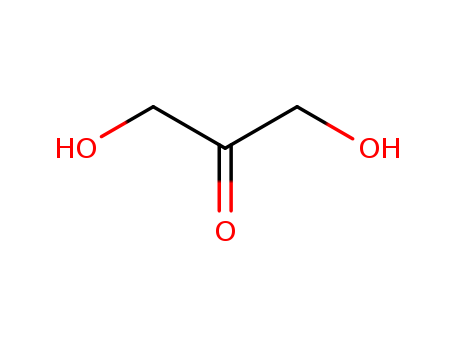
Product Name:1,3-Dihydroxyacetone
Molecular Formula:C3H6O3
Appearance:white powder
Purity:99%
Buy Buy High Quality 1,3-Dihydroxyacetone 96-26-4 Low Price
- Molecular Formula:C3H6O3
- Molecular Weight:90.0788
- Appearance/Colour:white powder
- Vapor Pressure:0.0358mmHg at 25°C
- Melting Point:75-80 °C
- Refractive Index:1.455
- Boiling Point:213.7 °C at 760 mmHg
- PKA:12.45±0.10(Predicted)
- Flash Point:97.3 °C
- PSA:57.53000
- Density:1.283 g/cm3
- LogP:-1.45990
1,3-Dihydroxyacetone(Cas 96-26-4) Usage
|
Chemical Properties |
white powder |
|
Occurrence |
A derivative of naturally occurring starch |
|
Uses |
1,3-Dihydroxyacetone can be used as artificial tanning agent. |
|
Preparation |
Usually produced commercially from Bacillus macerans or Bacillus circulans fermentation of starch or starch hydrolysate |
|
Definition |
ChEBI: A ketotriose consisting of acetone bearing hydroxy substituents at positions 1 and 3. The simplest member of the class of ketoses and the parent of the class of glycerones. |
|
Taste threshold values |
Reported to have a taste threshold value lower than that of sucrose with a detection level of 3.9 to 27 ppm and a recognition level of 11 to 52 ppm. |
|
General Description |
Dihydroxyacetone (DHA) is a browning ingredient widely used in cosmetics such as sunless tanning formulations. It participates in a chemical staining reaction called Milliard reaction in which it reacts with the amino groups of proteins to result in a mixture of high molecular weight pigments.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
|
Safety Profile |
Mutation data reported. When heated to decompositionit emits acrid smoke and irritating vapors. |
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products and perfumes and fragrances. Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid. |
InChI:InChI=1/C3H6O3/c4-1-3(6)2-5/h4-5H,1-2H2
96-26-4 Relevant articles
The selective oxidation of glycerol over metal-free photocatalysts: insights into the solvent effect on catalytic efficiency and product distribution
Fan, Mingming,Haryonob, Agus,Jiang, Pingping,Leng, Yan,Yue, Chengguang,Zhang, Pingbo
, p. 3385 - 3392 (2021/06/06)
Selective oxidation of glycerol to high ...
Earth-abundant manganese oxide nanoneedle as highly efficient electrocatalyst for selective glycerol electro-oxidation to dihydroxyacetone
Chiang, Chia-Ying,Tran, Giang-Son,Vo, Truong-Giang
, p. 139 - 148 (2021/10/07)
In this study, earth-abundant manganese ...
A proof of concept for cooperation from the quinone groups adjacent to N sites during the metal-free oxidation of glycerol by nitrogen-rich graphene oxide
Barlocco, Ilaria,Dogra, Ashima,Gupta, Neeraj,Sharma, Vinit,Villa, Alberto
supporting information, p. 19651 - 19654 (2021/11/12)
Glycerol is a key by-product in biodiese...
Assembly of platinum nanoparticles and single-atom bismuth for selective oxidation of glycerol
Huang, Ning,Jiang, Dong,Jiang, Pingping,Leng, Yan,Lu, Yubing,Tian, Jinshu,Yue, Chenguang,Zhang, Pingbo,Zhang, Zihao
supporting information, p. 25576 - 25584 (2021/12/07)
Selective oxidation of the secondary hyd...
96-26-4 Process route
-
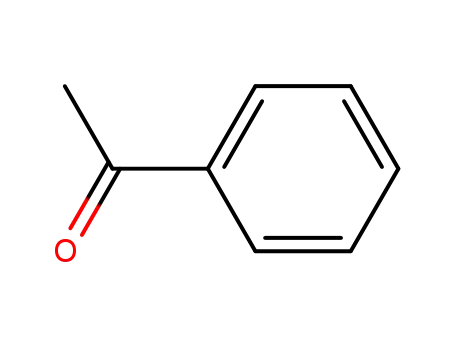
-
98-86-2
acetophenone

-
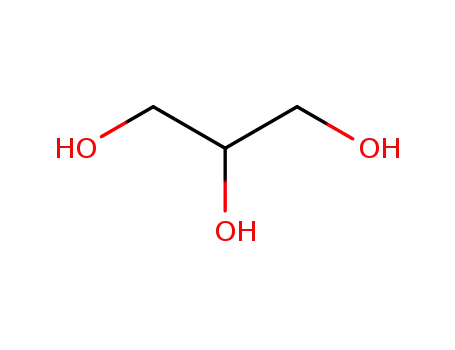
-
56-81-5,25618-55-7,64333-26-2,8013-25-0
glycerol

-
-
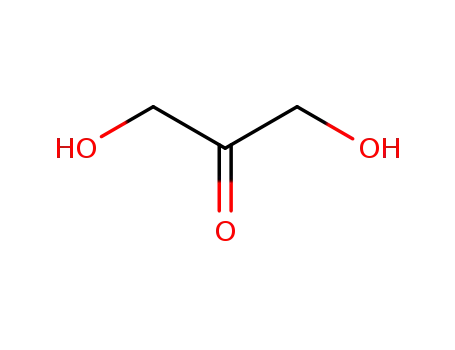
96-26-4,26776-70-5
dihydroxyacetone

-
-
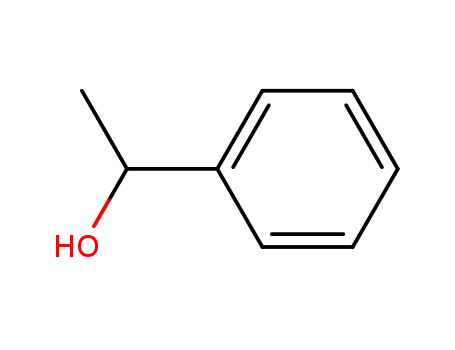
98-85-1,13323-81-4
1-Phenylethanol
| Conditions | Yield |
|---|---|
|
With
[IrCl(COD)(C3H2N2(3,4,5-trimethoxybenzyl)(n-Bu))]; potassium hydroxide;
at 120 ℃;
for 7h;
Inert atmosphere;
|
80% |
|
With
C40H50IrNP2;
at 120 ℃;
for 1h;
chemoselective reaction;
Inert atmosphere;
|
8 %Chromat. 6 %Chromat. |
-

-
98-86-2
acetophenone

-

-
56-81-5,25618-55-7,64333-26-2,8013-25-0
glycerol

-

-
96-26-4,26776-70-5
dihydroxyacetone

-

-
98-85-1,13323-81-4
1-Phenylethanol

-
![cis-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol](/upload/2023/8/62f964c7-156c-4f29-9362-470628b9cc3f.png)
-
cis-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol

-
![trans-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol](/upload/2023/8/577c5d87-c8d8-44e7-844f-7bbdb6821686.png)
-
trans-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol

-
-
C11H14O3

-
-
C11H14O3
| Conditions | Yield |
|---|---|
|
With
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)];
at 40 ℃;
for 1h;
Molecular sieve;
|
96-26-4 Upstream products
-
110-86-1
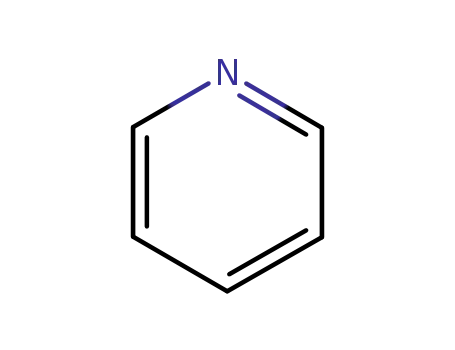
pyridine
-
56-82-6
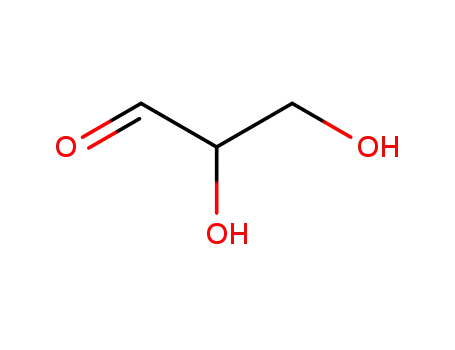
Glyceraldehyde
-
50-00-0
formaldehyd
-
50-99-7
D-glucose
96-26-4 Downstream products
-
822-36-6
4-methyl-1H-imidazole
-
78-98-8
2-oxopropanal
-
75-07-0
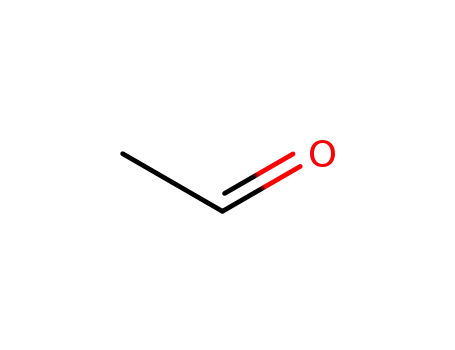
acetaldehyde
-
15719-64-9
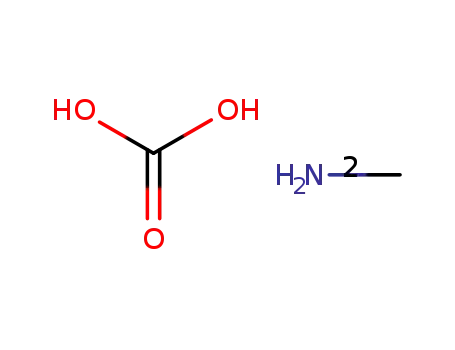
methylammonium carbonate