CasNo:446-72-0
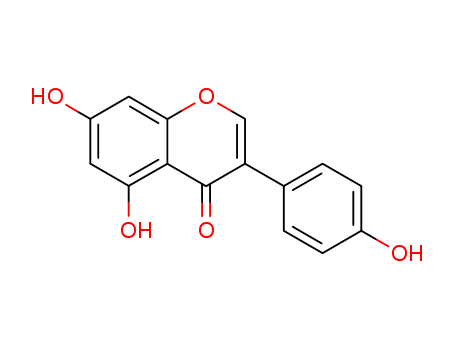
Product Name:Genistein
Molecular Formula:C15H10O5
Appearance:Yellow crystalline solid
Purity:99%
Chinese Factory In Bulk Supply 446-72-0, Factory Sells Genistein
- Molecular Formula:C15H10O5
- Molecular Weight:270.241
- Appearance/Colour:Yellow crystalline solid
- Vapor Pressure:6E-13mmHg at 25°C
- Melting Point:297-298 °C
- Refractive Index:1.732
- Boiling Point:555.5 °C at 760 mmHg
- PKA:6.51±0.20(Predicted)
- Flash Point:217.1 °C
- PSA:90.90000
- Density:1.548 g/cm3
- LogP:2.57680
Genistein(Cas 446-72-0) Usage
|
Description |
Genistein is a pale yellow dendritic needle-like powder with a melting point of 297-298°C. It is soluble in common organic solvents but almost insoluble in water. It can turn yellow when dissolved in dilute alkali. Genistein is a natural bioactive compound and is classified as an isoflavone. It is abundantly found in soy and soy-based products, such as tofu, and belongs to a group of compounds known as phytoestrogens. Genistein has been the focus of extensive research due to its potential health benefits and its impact on human physiology, particularly in the fields of cancer prevention, hormone-related diseases, and cardiovascular health. It has drawn attention for its natural origin, antioxidant properties, and potential to mimic the effects of human estrogen in certain biological processes. |
|
Uses |
Genistein's structural similarity to human estrogen allows it to act as a phytoestrogen. It has a weak estrogenic effect, which means it can interact with estrogen receptors and influence various biological processes in a manner similar to natural estrogen. Genistein is well-known for its role as a non-specific tyrosine kinase inhibitor, especially at pharmacological doses. Genistein exhibits antiangiogenic properties, meaning it can inhibit the formation of new blood vessels, a process crucial for tumor growth. Additionally, it has antioxidant activity, reducing oxidative stress and the impact of free radicals in cells. |
|
Chemical Properties |
Yellow Crystalline Solid |
InChI:InChI=1/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
446-72-0 Relevant articles
Biological properties of genistein. A review of in vitro and in vivo data
K Polkowski, AP Mazurek
, Acta Pol. Pharm, 2000
The great interest that has focused on genistein led to the identification of numerous … after purified genistein administration at higher doses. The main genistein advantage as a …
Deglycosylation of isoflavonoid glycosides from maackia amurensis cell culture by β-D-glucosidase from littorina sitkana hepatopancrease
Kusaikin,Zakharenko,Ermakova,Veselova,Grigoruk,Fedoreev,Zvyagintseva
, p. 197 - 200 (2011)
Maakia amurensis (strain A-18) cell cult...
Understanding genistein in cancer: The “good” and the “bad” effects: A review
Maria Russo a 1, Gian Luigi Russo a 1, Maria Daglia b 1, Pandima Devi Kasi c, Sakthivel Ravi c, Seyed Fazel Nabavi d, Seyed Mohammad Nabavi d
, Food Chemistry Volume 196, 1 April 2016, Pages 589-600
A plethora of evidence supports the in vitro and in vivo anticancer effects of genistein, a soybean isoflavone. Major tumors affected by genistein here reviewed are breast, prostate, colon, liver, ovarian, bladder, gastric, brain cancers, neuroblastoma and chronic lymphocytic leukemia.
A novel method for the highly efficient biotransformation of genistein from genistin using a high-speed counter-current chromatography bioreactor
Wang, Daijie,Khan, Muhammad Shafiq,Cui, Li,Song, Xiangyun,Zhu, Heng,Ma, Tianyu,Li, Xiaoyu,Sun, Rong
, p. 4892 - 4899 (2019)
Genistein, an important soybean isoflavo...
Identification of ortho catechol-containing isoflavone as a privileged scaffold that directly prevents the aggregation of both amyloid β plaques and tau-mediated neurofibrillary tangles and its in vivo evaluation
Do, Ji Min,Gee, Min Sung,Inn, Kyung-Soo,Kim, Jong-Ho,Kim, Nam Kwon,Kim, Nam-Jung,Lee, Hyun Woo,Lee, Jong Kil,Seo, Min-Duk,Seong, Ji Hye,Son, Seung Hwan,Yoo, Hyung-Seok,Yoo, Ji-Na
, (2021/07/01)
In this study, polyhydroxyisoflavones th...
446-72-0 Process route
-
![7-((2S,3R,4R)-3,4-Dihydroxy-4-hydroxymethyl-tetrahydro-furan-2-yloxy)-3-[4-((2R,3S,4S)-3,4-dihydroxy-4-hydroxymethyl-tetrahydro-furan-2-yloxy)-phenyl]-5-hydroxy-chromen-4-one](/upload/2023/8/9cca13fd-f6d7-48f0-9f36-dafeb7da0d11.png)
- 78694-77-6
7-((2S,3R,4R)-3,4-Dihydroxy-4-hydroxymethyl-tetrahydro-furan-2-yloxy)-3-[4-((2R,3S,4S)-3,4-dihydroxy-4-hydroxymethyl-tetrahydro-furan-2-yloxy)-phenyl]-5-hydroxy-chromen-4-one

-
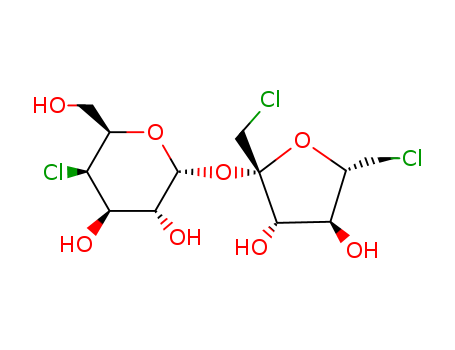
- 639-97-4,6477-44-7,42927-70-8
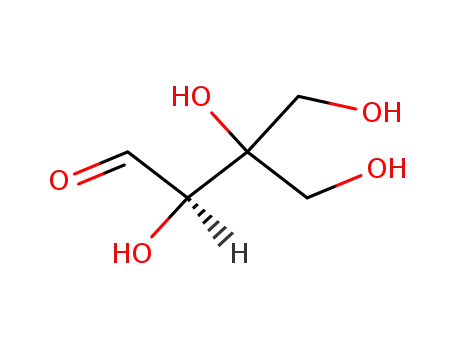
D-apiose

-
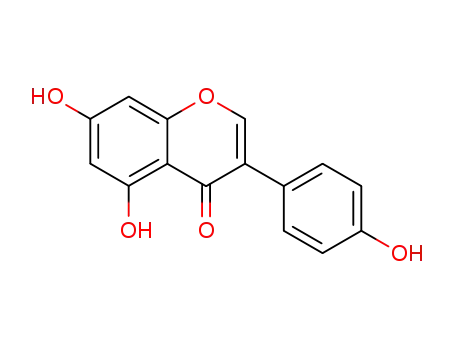
- 446-72-0
5,7-Dihydroxy-3-(4-hydroxy-phenyl)-chromen-4-on
| Conditions | Yield |
|---|---|
|
With sulfuric acid; Heating;
|
-
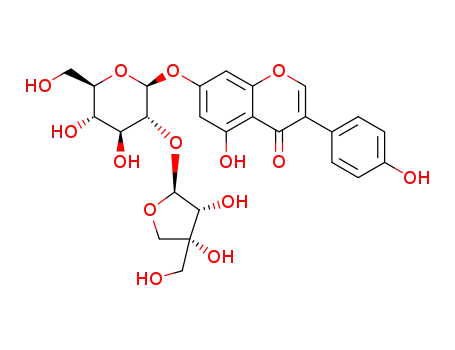
- 1673575-50-2
chevalierinoside C

-
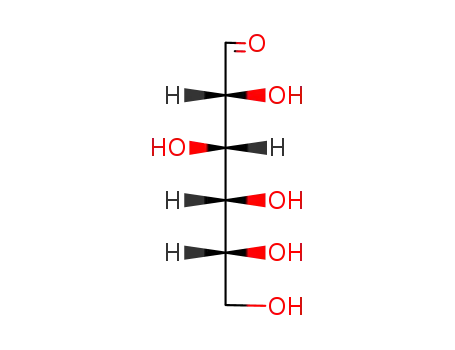
- 50-99-7
D-glucose

-

- 446-72-0
5,7-Dihydroxy-3-(4-hydroxy-phenyl)-chromen-4-on
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; at 80 ℃; for 3h;
|
2 mg |
446-72-0 Upstream products
-
491-80-5
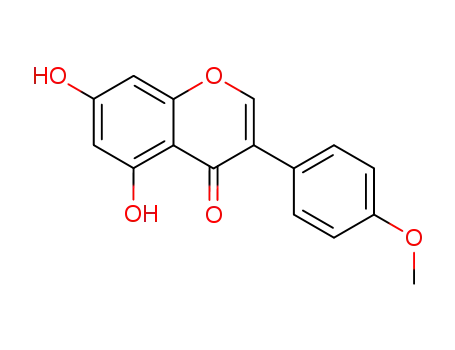
5,7-Dihydroxy-3-(4-methoxy-phenyl)-chromen-4-on
-
22151-32-2
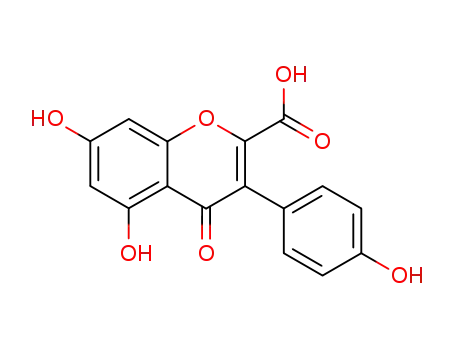
5,7-dihydroxy-3-(4-hydroxy-phenyl)-4-oxo-4H-chromene-2-carboxylic acid
-
290-87-9
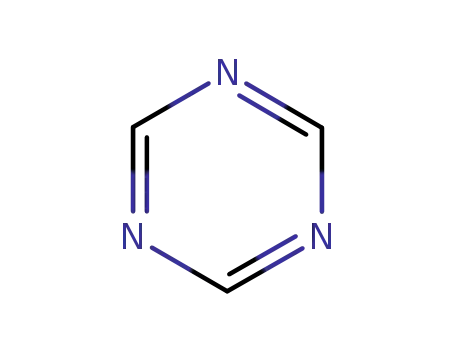
1,3,5-Triazine
-
15485-65-1
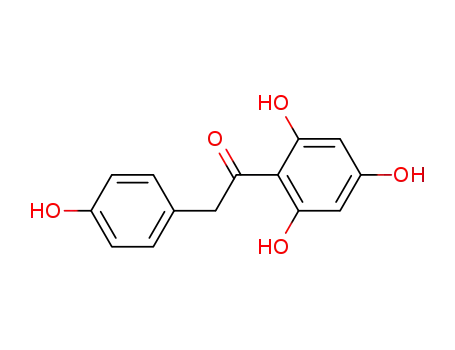
2,4,4',6-tetrahydroxydeoxybenzoin
446-72-0 Downstream products
-
34086-51-6
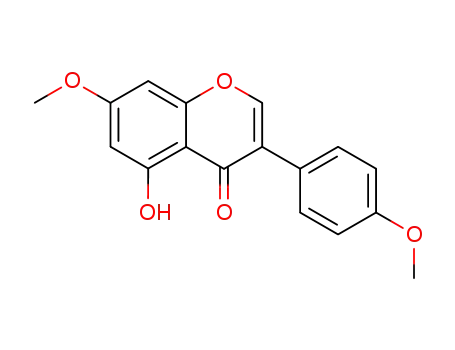
4',7-dimethoxy-5-hydroxyisoflavone
-
1162-82-9
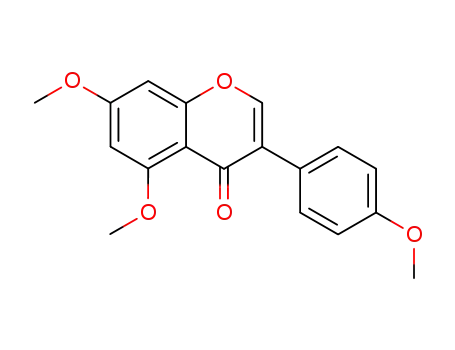
4',5,7-trimethoxyisoflavone
-
552-59-0
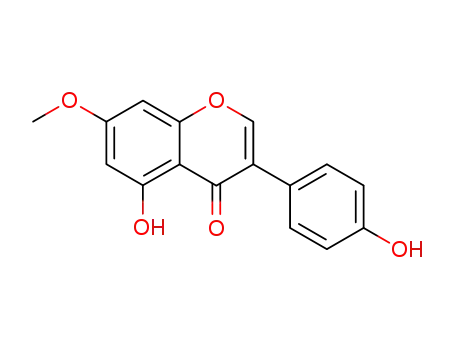
prunetin
-
874532-59-9
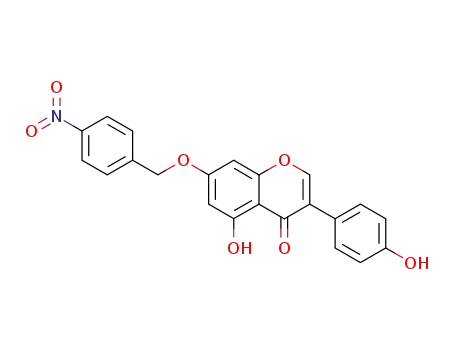
5-hydroxy-3-(4-hydroxy-phenyl)-7-(4-nitro-benzyloxy)-chromen-4-one




![1H-Benzimidazole-1-ethanamine, 5-amino-N,N-diethyl-2-[(4-methoxyphenyl)methyl]-](/upload/2023/8/3cbf17a1-ba87-4940-bedf-eb1eedefc485.png)