CasNo:79-94-7
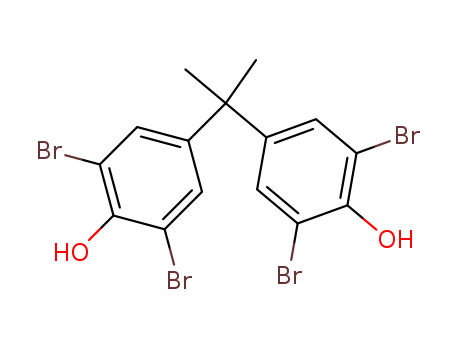
Product Name:Tetrabromobisphenol A
Molecular Formula:C15H12Br4O2
Appearance:white to pale cream or pale yellow crystalline
Purity:99%
Buy Quality High Purity 99% Tetrabromobisphenol A 79-94-7 with Safe Shipping
- Molecular Formula:C15H12Br4O2
- Molecular Weight:543.875
- Appearance/Colour:white to pale cream or pale yellow crystalline
- Vapor Pressure:0Pa at 20℃
- Melting Point:178-181 °C(lit.)
- Refractive Index:1.5000 (estimate)
- Boiling Point:417.9 °C at 760 mmHg
- PKA:8.50±0.10(Predicted)
- Flash Point:206.6 °C
- PSA:40.46000
- Density:2.057 g/cm3
- LogP:6.47370
Tetrabromobisphenol A(Cas 79-94-7) Usage
|
Chemical Properties |
Tetrabromobisphenol A is a white to pale cream or pale yellow crystalline with a moderately high molecular weight, low water solubility, and moderately high lipophilicity (as indicated by log Kow). Only about 4% of the particles are <15 μm in diameter, and thus, little (<4%) is expected to be respirable (<10 μm in diameter) and absorbed from the lung after inhalation exposure. Tetrabromobisphenol A (TBBPA) is a brominated flame retardant used in a variety of reactive and additive applications. It is reacted (i.e., covalently bound) with epoxy, vinyl esters, and polycarbonate systems (e.g., high impact polystyrene (HIPS), and is used as an additive in acrylonitrile-butadiene-styrene (ABS) thermoplastic resins (Albemarle, 1999). Its primary application is in printed wire boards (PWBs) as a reactive flame retardant (BSEF, 2012). |
|
Uses |
tetrabromobisphenol A is widely used as a reactive flame retardant to produce a bromine-containing epoxy resin and polycarbonate, and as intermediates for the synthesis of other complex flame retardant, also as an additive flame retardant for ABS, HIPS, unsaturated polyester rigid polyurethane foams, adhesives and coatings. |
|
Definition |
ChEBI: Tetrabromobisphenol A is a bromobisphenol that is 4,4'-methanediyldiphenol in which the methylene hydrogens are replaced by two methyl groups and the phenyl rings are substituted by bromo groups at positions 2, 2', 6 and 6'. It is a brominated flame retardant. It is a brominated flame retardant and a bromobisphenol. It is functionally related to a bisphenol A. |
|
Application |
The main use of TBBPA is as a reactive flame retardant in epoxy resins for printed circuit boards in computers, telecommunications equipment, industrial controls and automotive electronics. Both hydroxyl groups on TBBPA can be reacted with epichlorohydrin under basic conditions to form the diglycidyl ether, which is widely used in epoxy resin formulations. TBBPA is also used in polycarbonate and ether polyester resins and is used as a chemical intermediate for the synthesis of tetra-bromobisphenol A allyl ether, -bis(2-hydroxyethyl ether), -carbonate oligomer, and -diglycidyl ether. TBBPA is also used as a flame retardant in plastics, paper, and textiles, and as a plasticizer in adhesives and coatings. Being covalently bound to the polymer limits exposure to unbound excess chemical used in the manufacturing process. |
|
General Description |
White powder. A monomer for flame-retardant epoxy, polyester and polycarboante resins. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Tetrabromobisphenol A is monomer. |
|
Hazard |
Moderately toxic by inhalation and skincontact. An eye irritant. |
|
Fire Hazard |
Tetrabromobisphenol A is nonflammable. |
|
Flammability and Explosibility |
Nonflammable |
|
Toxicity evaluation |
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant that has been associated with kidney toxicity in newborn rats. TBBPA is similar in structure to the thyroid hormone T4 and has been found to compete with T4 in binding to proteins in the blood which reduce overall blood serum levels of thyroid hormones.Tetrabromobisphenol A is classified as hazard statements (H) H400/H410, which means that it is toxic to aquatic biota, causing long-term changes in these organisms.TBBPA is included on Washington State's PBT (Persistent, Bioaccumulative and Toxic) Rule of chemicals.TBBPA is on the Proposition 65 list because it can cause cancer.A National Toxicology Program two-year bioassay study reported that there was clear cancer development in female rats that were exposed to TBBPA, and some evidence in male mice. |
InChI:InChI=1/C15H12Br4O2/c1-15(2,7-3-9(16)13(20)10(17)4-7)8-5-11(18)14(21)12(19)6-8/h3-6,20-21H,1-2H3
79-94-7 Relevant articles
Instantaneous, facile and selective synthesis of tetrabromobisphenol a using potassium tribromide: An efficient and renewable brominating agent
Kumar, Lalit,Sharma, Vivek,Mahajan, Tanu,Agarwal
, p. 174 - 179 (2010)
An instantaneous method for the brominat...
Electrophilic bromination in flow: A safe and sustainable alternative to the use of molecular bromine in batch
Van Kerrebroeck, Reinout,Naert, Pieter,Heugebaert, Thomas S.A.,D’hooghe, Matthias,Stevens, Christian V.
, (2019/06/10)
Bromination reactions are crucial in tod...
Electrochemical synthesis of quinones and other derivatives in biphasic medium
Shanmugam,Kulangiappar,Ramaprakash,Vasudevan,Senthil Kumar,Velayutham,Raju
, p. 2294 - 2297 (2017/05/19)
Electrochemical synthesis of quinones ha...
Hexamethonium bis(tribromide) (HMBTB) a recyclable and high bromine containing reagent
Paul, Bappi,Bhuyan, Bishal,Purkayastha, Debraj D.,Dhar, Siddhartha S.,Patel, Bhisma K.
, p. 5646 - 5650 (2015/09/21)
A recyclable and high bromine containing...
79-94-7 Process route
-
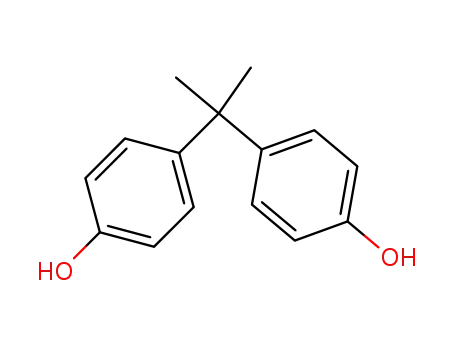
- 80-05-7
BPA

-
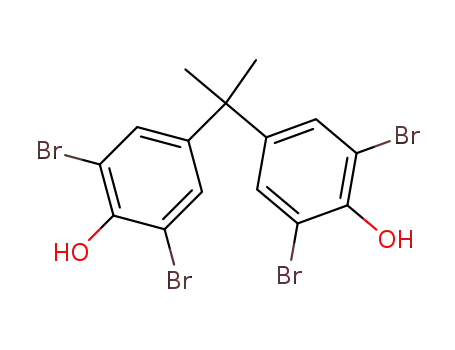
- 79-94-7
Tetrabromobisphenol A
| Conditions | Yield |
|---|---|
|
With potassium tribromide; In water; acetonitrile; at 30 ℃; for 0.0833333h;
|
99% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In tetrachloromethane; water; at 10 ℃; for 4.5h;
|
98.28% |
|
With dihydrogen peroxide; bromine; In dichloromethane; water;
|
96% |
|
With sulfuric acid; sodium bromide; In dichloromethane; water; at 15 ℃; for 2.98h; Electrolysis;
|
95% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 4.5h;
|
93% |
|
With benzyltriphenylphosphonium tribromide; calcium carbonate; In methanol; dichloromethane; at 20 ℃; for 1h;
|
92% |
|
With sodium bromate; hydrogen bromide; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 4.75h;
|
92.11% |
|
With dihydrogen peroxide; bromine; In dichloromethane; water; at 38 - 41 ℃; for 1.75 - 8.75h; Product distribution / selectivity; Heating / reflux;
|
92.9% |
|
With 1-benzyl-1-aza-4-azoniabicyclo<2.2.2>octane bromide; calcium carbonate; In methanol; at 20 ℃; for 1.16667h;
|
90% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 4.5h;
|
90.44% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 3.5h;
|
85.4% |
|
With sodium hypochlorite; hydrogen bromide; sodium sulfite; In diethyl ether; water; at 20 ℃; for 0.0416667h; Flow reactor;
|
83% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 4.5h;
|
66.61% |
|
With hexamethonium bis(tribromide); In neat (no solvent); for 1h; regioselective reaction; Green chemistry;
|
60% |
|
With bromine; acetic acid; at 45 ℃; for 2h;
|
56% |
|
With hydrogenchloride; sodium bromate; sodium dodecyl-sulfate; sodium bromide; In dichloromethane; water; at 10 ℃; for 3.5h;
|
52.86% |
|
With bromine; acetic acid;
|
|
|
With bromine; acetic acid; at 24 ℃;
|
|
|
With dihydrogen peroxide; bromine; In dichloromethane; water; Product distribution / selectivity;
|
-
methanolic TBA solution containing excess bromine
-
methanolic TBA solution containing excess bromine

-

- 79-94-7
Tetrabromobisphenol A
| Conditions | Yield |
|---|---|
|
With sodium sulfite; In water; at 30 ℃; for 12h; Purification / work up;
|
|
|
With hydrazine; In water; at 30 ℃; Purification / work up;
|
|
|
With sulfur dioxide; In water; Purification / work up;
|
|
|
In water; Purification / work up;
|
79-94-7 Upstream products
-
80-05-7

BPA
79-94-7 Downstream products
-
608134-64-1
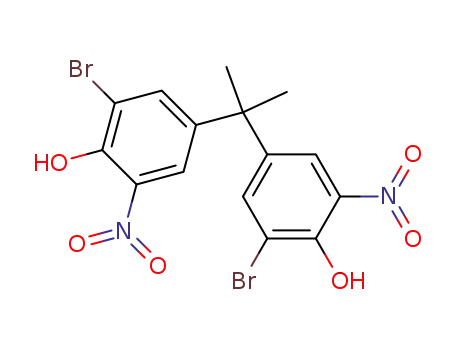
2,2'-dibromo-6,6'-dinitro-4,4'-isopropylidene-di-phenol
-
33798-02-6
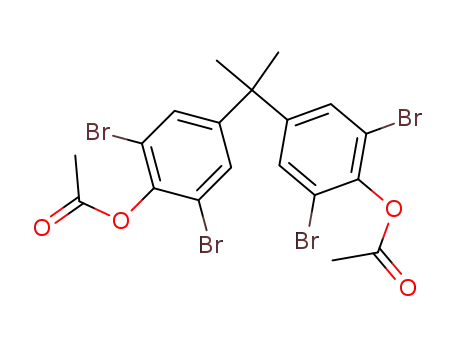
2,2-bis-(4-acetoxy-3,5-dibromo-phenyl)-propane
-
98572-84-0
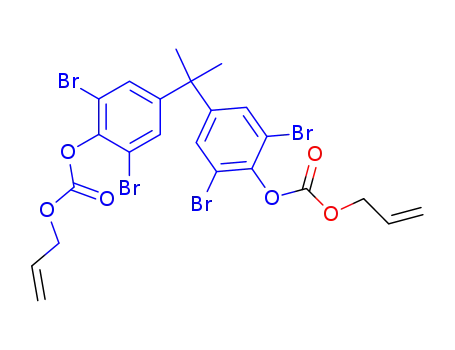
2,2-bis-(4-allyloxycarbonyloxy-3,5-dibromo-phenyl)-propane
-
38578-49-3
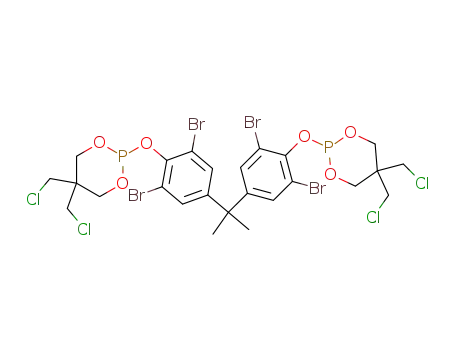
C25H26Br4Cl4O6P2