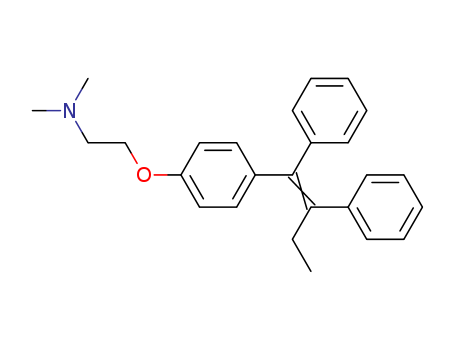
CasNo:10540-29-1
Product Name:Tamoxifen Citrate
Molecular Formula:C26H29NO
Appearance:White crystalline solid
Purity:99%
Wholesale Factory Supply Tamoxifen Citrate 10540-29-1 with Cheapest Price. We supply high quality Tamoxifen (CAS 10540-29-1), in stock, factory directly supply to clients, lower prices, more competitiveness.
What is the Tamoxifen ?
Tamoxifen is White crystalline solid, while it's Molecular Formula is C26H29NO. Tamoxifen has been used to facilitate the recombination of ect2flox allele in mouse organs91. It has also been used to study its effect on lipopolysaccharide (LPS)-induced microglial activation92.
What is the CAS number for Tamoxifen ?
The CAS number of Tamoxifen is 10540-29-1.
More information of Tamoxifen 10540-29-1 are:
|
Synonyms |
Ethanamine,2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethyl-, (Z)-;Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-butenyl]phenoxy]-N,N-dimethyl-(9CI);Ethylamine, 2-[p-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethyl-, (Z)-(8CI);(Z)-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine;ICI47699;Mammaton;Novaldex;Z-Tamoxifen;trans-Tamoxifen;? Tamoxifen; |
|
CAS Number |
10540-29-1 |
|
Molecular Formula |
C26H29NO |
|
Molecular Weight |
371.522 |
|
Density |
1.042 g/cm3 |
|
Melting Point |
97-98 °C(lit.) |
|
Boiling Point |
482.3 °C at 760 mmHg |
|
Flash Point |
140 °C |
|
HS CODE |
29221990 |
|
PSA |
12.47000 |
|
LogP |
5.99610 |
|
Pka |
pKa 8.71(H2O t = 25 I = 0.025) (Uncertain) |
What is Tamoxifen (10540-29-1) used for?
Tamoxifen citrate is an antiestrogen for peroral breast cancer treatment. It is used to treat breast cancer. It is also used to reduce the chances of breast cancer in high-risk patients.
In 1966, ICI Pharmaceuticals (now AstraZeneca) first synthesized tamoxifen in the hope of developing a morning-after contraceptive pill. The UK patent for this compound was in place in 1962, whereas the US patent was repeatedly denied until the 1980s. Tamoxifen was approved for a fertility treatment but it was not proven as useful in regulating human contraception. Even though there was a link between estrogen and breast cancer, developing a cancer treatment was not a priority at the time. In 1971, the first clinical study showed a convincing effect of tamoxifen in treating advanced breast cancer. From 1971 to 1977, this drug was neither clinically nor financially remarkable. In 1980s, however, publications first showed that tamoxifen, in addition to chemotherapy, improved survival for patients with early stage breast cancer. In 1998, the meta-analysis by the Oxford-based Early Breast Cancer Trialists’ Collaborative Group showed that tamoxifen did indeed save lives in early breast cancer. In 2001, tamoxifen sales were over $1.024 billion. Since the expiration of the patent in 2002, it is now widely available as a generic drug. By 2004, tamoxifen was the best selling hormonal drug for the treatment of breast cancer.
InChI:InChI=1/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25+
Articles related to Tamoxifen:
|
Article |
Source and Abstract |
| Tamoxifen citrate loaded amphiphilic β-cyclodextrin nanoparticles: In vitro characterization and cytotoxicity |
Cytotoxic efficacy of tamoxifen citrate loaded nanospheres and nanocapsules was determined against MCF-7 cells and tamoxifen citrate incorporated in amphiphilic β-cyclodextrin nanoparticles was found to be cytotoxic and effective against this cell line. , Journal of Controlled Release Volume 104, Issue 3, 2 June 2005, Pages 489-496 |
| A prospective randomized trial comparing clomiphene citrate with tamoxifen citrate for ovulation induction |
To compare the rates of ovulation and pregnancy after tamoxifen citrate (TMX) or clomiphene citrate (CC) among anovulatory women with infertility. , Fertility and Sterility Volume 75, Issue 5, May 2001, Pages 1024-1026 |
|
Opportune gem-Silylborylation of Carbonyl Compounds: A Modular and Stereocontrolled Entry to Tetrasubstituted Olefins |
La Cascia, Enrico,Cuenca, Ana B.,Fernández, Elena , p. 18737 - 18741 (2016) |
Wholesale Factory Supply Tamoxifen Citrate 10540-29-1 with Cheapest Price
Henan Xiaguo Biotechnology Co., Ltd. is a quality supplier and manufacturer of Tamoxifen . You can buy high quality, low price Tamoxifen 10540-29-1 here. Contact us.