CasNo:23593-75-1
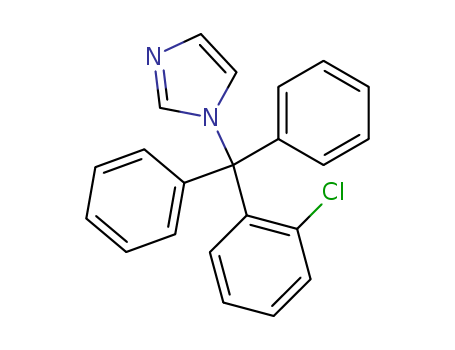
Product Name:clotrimazole
Molecular Formula:C22H17ClN2
Appearance:Crystalline
Purity:99%
Quality Factory Supply clotrimazole, Offer 23593-75-1 with Reasonable Price
- Molecular Formula:C22H17ClN2
- Molecular Weight:344.843
- Appearance/Colour:Crystalline
- Vapor Pressure:5.42E-09mmHg at 25°C
- Melting Point:147-149 °C
- Refractive Index:1.616
- Boiling Point:482.3 °C at 760 mmHg
- PKA:pKa 4.7(EtOH 50%aq ) (Uncertain)
- Flash Point:245.5 °C
- PSA:17.82000
- Density:1.13 g/cm3
- LogP:5.37670
Clotrimazole(Cas 23593-75-1) Usage
|
Chemical Properties |
Clotrimazole is a white or colorless crystalline powder with a melting point of 147-149 °C. It is soluble in ethanol, acetone, and chloroform but nearly insoluble in water. It is odorless and tasteless but rapidly decomposes in an acid solution. Clotrimazole hydrochloride has a melting point of 159 °C. |
|
Description |
Clotrimazole is an antifungal agent, specifically an imidazole antifungal, that exhibits broad-spectrum activity against various forms of fungi. It is used for the treatment of fungal infections, including those affecting the skin, mouth, and vaginal mucosa. |
|
Originator |
Canesten,Bayer,UK,1973 |
|
Uses |
Clotrimazole is used topically for various fungal infections, including oral, skin, and vaginal infections caused by C. albicans. It is also employed for cutaneous dermatophyte infections. Clotrimazole is available in the form of 1% vaginal cream and tablets (100 mg and 500 mg) for the treatment of vulvovaginal candidiasis. |
|
Brand name |
Gyne- Lotrimin (Schering-Plough); Gynix (Teva); Lotrimin (Schering-Plough); Mycelex (Bayer). |
|
Therapeutic Function |
Antifungal |
|
Veterinary Drugs and Treatments |
Topical clotrimazole has activity against dermatophytes and yeasts; it may be useful for localized lesions associated with Malassezia. It is not very effective in treating dermatophytosis in cats. Clotrimazole inhibits the biosynthesis of ergosterol, a component of fungal cell membranes leading to increased membrane permeability and probable disruption of membrane enzyme systems. |
InChI:InChI=1/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H
23593-75-1 Relevant articles
Clotrimazole: A Review of its Antifungal Activity and Therapeutic Efficacy
Phyllis R. Sawyer, R. N. Brogden, K. M. Pinder, T. M. Speight & G. S. Avery
, Drugs volume 9, pages424–447 (1975)
Candidal septicemia and urinary and pulmonary candidiasis have been cured with oral clotrimazole therapy. Results in other types of serious fungal infections, including pulmonary aspergillosis, have been disappointing. A limiting factor in oral clotrimazole therapy is the high incidence of gastro-intestinal disturbances and neurological reactions.
Clotrimazole inhibits cell proliferation in vitro and in vivo
Laura R. Benzaquen, Carlo Brugnara, H. Randolph Byers, Sebastiano Gattoni-Celli & José A. Halperin
, Nature Medicine volume 1, pages534–540 (1995)
The antimycotic drug clotrimazole (CLT) has been shown to inhibit movement of Ca2+ and K+ across the plasma membrane. Our results show that CLT inhibits the rate of cell proliferation of normal and cancer cell lines in a reversible and dose-dependent manner in vitro.
Fluorinated Alcohol-Promoted Reaction of Chlorohydrocarbons with Diverse Nucleophiles for the Synthesis of Triarylmethanes and Tetraarylmethanes
Yu, Liping,Li, Shuai-Shuai,Li, Weina,Yu, Shitao,Liu, Qing,Xiao, Jian
, p. 15277 - 15283 (2019/01/04)
This article reports an efficient synthe...
23593-75-1 Process route
-
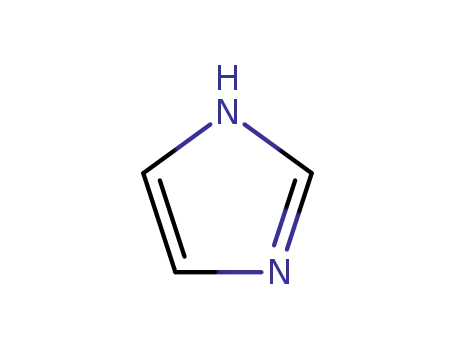
- 288-32-4
1H-imidazole

-
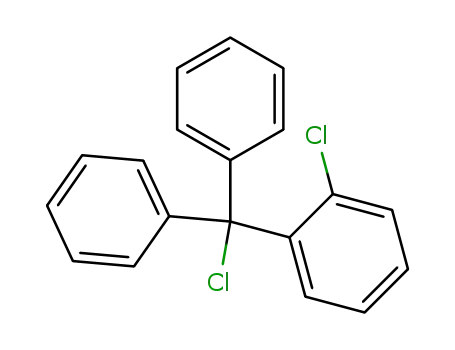
- 42074-68-0
2-chlorotrityl chloride

-
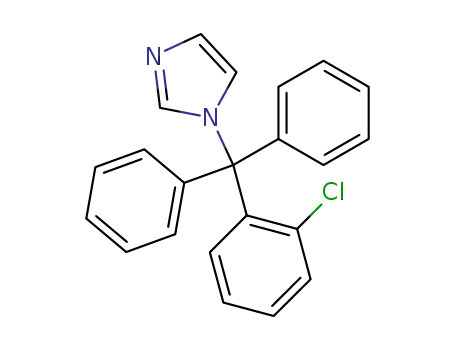
- 23593-75-1
clotrimazole
| Conditions | Yield |
|---|---|
|
With sodium carbonate; at 25 ℃; for 48h; Reagent/catalyst; Solvent; Concentration; Green chemistry;
|
92% |
|
With sodium carbonate; at 20 ℃; for 48h; Sealed tube;
|
92% |
|
With triethylamine; In acetonitrile; Heating;
|
|
|
With triethylamine; In toluene; at 20 - 50 ℃; for 3h; Temperature; Solvent; Inert atmosphere;
|
-

- 288-32-4
1H-imidazole

-
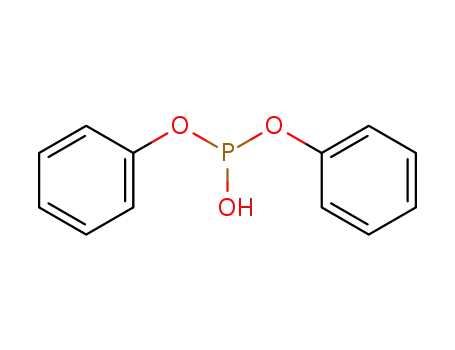
- 102-10-3
diphenyl phosphite

-
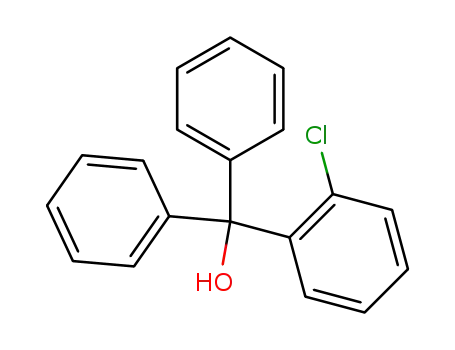
- 66774-02-5
o-chlorotriphenylmethanol

-
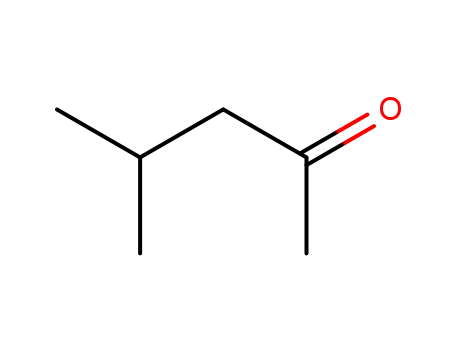
- 108-10-1
4-methyl-2-pentanone

-

- 23593-75-1
clotrimazole
| Conditions | Yield |
|---|---|
|
With sodium hydroxide;
|
100% |
|
With sodium hydroxide;
|
77% |