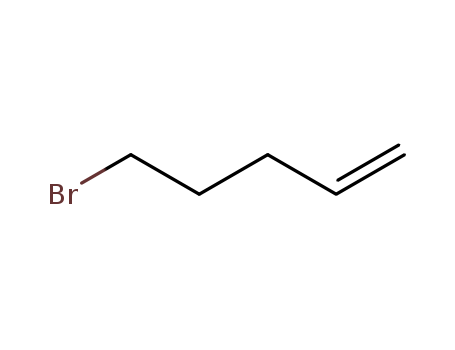CasNo:1119-51-3
Product Name:5-Bromo-1-pentene
Molecular Formula:C5H9Br
Appearance:clear colourles to yellow liquid
Purity:99%
Hot Sale! Factory Supply High Purity 5-Bromo-1-pentene 1119-51-3 Efficient Shipping
- Molecular Formula:C5H9Br
- Molecular Weight:149.03
- Appearance/Colour:clear colourles to yellow liquid
- Vapor Pressure:14.3mmHg at 25°C
- Melting Point:-106.7°C (estimate)
- Refractive Index:n20/D 1.463(lit.)
- Boiling Point:126.2 °C at 760 mmHg
- Flash Point:31 °C
- PSA:0.00000
- Density:1.248 g/cm3
- LogP:2.34750
5-Bromo-1-pentene(Cas 1119-51-3) Usage
| Description | 5-Bromo-1-pentene is a chemical compound known for its use in various organic syntheses. It is a clear colorless to yellow liquid at room temperature. It can be synthesized through various chemical reactions. Its role as a starting material in diverse syntheses demonstrates its versatility in organic chemistry. |
|
Uses |
5-Bromo-1-pentene has been employed in stereoselective synthesis processes. For example, it has been used in the synthesis of 7α-(3-carboxypropyl) estradiol, a steroid derivative. It has been used in the preparation of thioacetate derivatives of sialic acid, which contain thioglycosidic linkages. |
InChI:InChI=1/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
1119-51-3 Relevant articles
Monochloro-substituted phenyl carbamoylated β-cyclodextrins as π-acid chiral stationary phases for high-performance liquid chromatography
Z Wu Bai, C Bun Ching, S Choon Ng
, Chromatographia, 2003
First, b-cyclodextrin was reacted with 5-bromo-1pentene in dry DMF at room temperature to give 6A-O-(4¢-pentenyl)-b-cyclodextrin. The remaining twenty hydroxyl groups of this compound …
Cobalt-Mediated η5-Pentadienyl/Alkyne [5 + 2] Cycloaddition Reactions: Substitution Effects, Bicyclic Synthesis, and Photochemical η4-Cycloheptadiene Demetalation
Ylijoki, Kai E. O.,Kirk, Andrew D.,B?cklein, Sebastian,Witherell, Ross D.,Stryker, Jeffrey M.
, p. 3335 - 3357 (2015)
The preparation of seven-membered carboc...
Novel synthetic method 5-bromo-1-pentene (by machine translation)
-
Paragraph 0021; 0031-0042, (2020/06/30)
N, N - dimethylformamide is used as a st...
Formal Bromine Atom Transfer Radical Addition of Nonactivated Bromoalkanes Using Photoredox Gold Catalysis
Zidan, Montserrat,McCallum, Terry,Swann, Rowan,Barriault, Louis
supporting information, p. 8401 - 8406 (2020/11/03)
Organic transformations mediated by phot...
1119-51-3 Process route
-
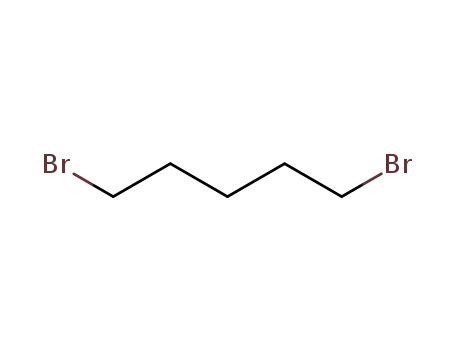
- 111-24-0
1,5-dibromo-pentane

-
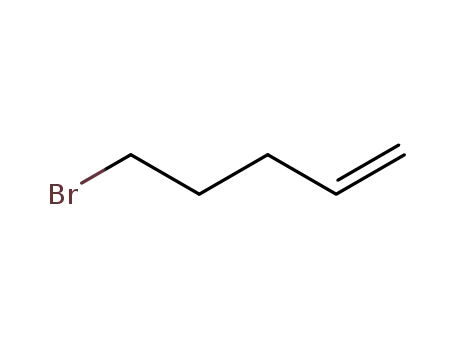
- 1119-51-3
bromopentene
| Conditions | Yield |
|---|---|
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; In N,N-dimethyl-formamide; at 140 ℃; for 4h; Time; Temperature; Reagent/catalyst; Large scale;
|
80.1% |
|
With potassium tert-butylate; In tetrahydrofuran; toluene; at 0 ℃; for 0.5h;
|
69% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 195 - 220 ℃;
|
60% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 195 - 230 ℃;
|
59% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 220 ℃; for 0.0833333h;
|
57% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 180 ℃;
|
54% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 195 - 200 ℃;
|
47% |
|
With 18-crown-6 ether; potassium hydroxide; at 200 ℃; for 7h; under 270.027 Torr;
|
47% |
|
In N,N,N,N,N,N-hexamethylphosphoric triamide; 195 deg C then 220 deg C;
|
46% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 205 ℃; for 0.0833333h;
|
44% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 195 - 220 ℃; for 0.0833333h;
|
42% |
|
With 18-crown-6 ether; potassium tert-butylate; In diethyl ether; for 1h;
|
37% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; at 195 ℃;
|
|
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; Heating;
|
|
|
With 18-crown-6 ether; potassium tert-butylate; In diethyl ether;
|
-
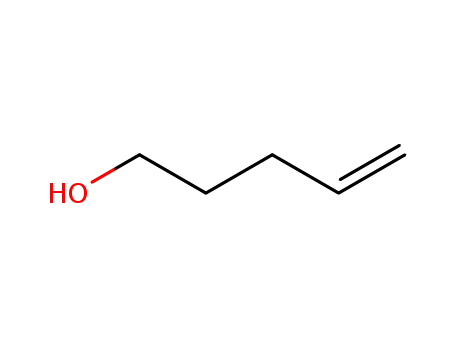
- 821-09-0
n-Pent-4-enyl alcohol

-
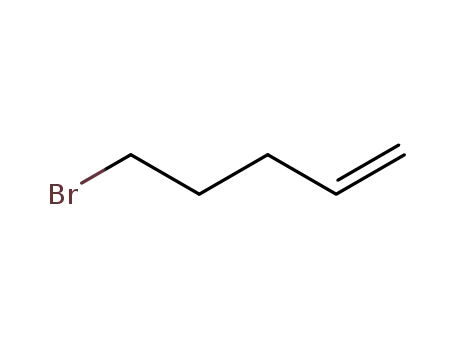
- 1119-51-3
bromopentene
| Conditions | Yield |
|---|---|
|
With carbon tetrabromide; triphenylphosphine; In dichloromethane; at 0 - 20 ℃; for 2.5h;
|
80% |
|
With pyridine; bromine; triphenylphosphine; In benzene;
|
73% |
|
With pyridine; phosphorus tribromide; 1.) -5 deg C, 30 min, 2.) RT, 2 h;
|
60% |
|
n-Pent-4-enyl alcohol; With phosphorus tribromide; In diethyl ether; at -30 - 20 ℃; for 16.5h;
With sodium bromide; In diethyl ether; for 21h;
|
60% |
|
With phosphorus tribromide; In n-heptane; at -10 ℃; for 2h;
|
55% |
|
With phosphorus tribromide; In Petroleum ether; at -20 ℃;
|
46% |
|
With pyridine; phosphorus tribromide; at -30 - -25 ℃; weniger gut bei Kuehlung mit Eis;
|
|
|
With pyridine; phosphorus tribromide;
|
|
|
|
|
|
With phosphorus tribromide;
|
|
|
With pyridine; phosphorus tribromide; at -5 ℃; for 0.25h;
|
|
|
With pyridine; phosphorus tribromide; at -30 - -25 ℃; for 1.16667h;
|
|
|
With pyridine; phosphorus tribromide; In diethyl ether;
|
|
|
With pyridine; triphenylphosphine dibromide 1:1 addition complex; In dichloromethane;
|
|
|
With bromine; triphenylphosphine; Yield given; 1.) CH2Cl2, 15 min., 2.) pyridine, ambient. temp., 1 h;
|
|
|
With pyridine; phosphorus tribromide; In Petroleum ether; at 0 ℃; for 1h;
|
|
|
With pyridine; phosphorus tribromide;
|
|
|
With N-Bromosuccinimide; triphenylphosphine; In N,N-dimethyl-formamide; at 20 ℃;
|
|
|
Multi-step reaction with 2 steps
1: KOH / diethyl ether / 2 h / 10 - 15 °C
2: LiBr / acetone / 1 h / Heating
With potassium hydroxide; lithium bromide; In diethyl ether; acetone;
|
|
|
With phosphorus tribromide; In diethyl ether; at -15 - 20 ℃; for 1.5h; Reflux; Inert atmosphere; Schlenk technique;
|
|
|
With carbon tetrabromide; triphenylphosphine; In dichloromethane;
|